Mesoblast Remestemcel L Covid
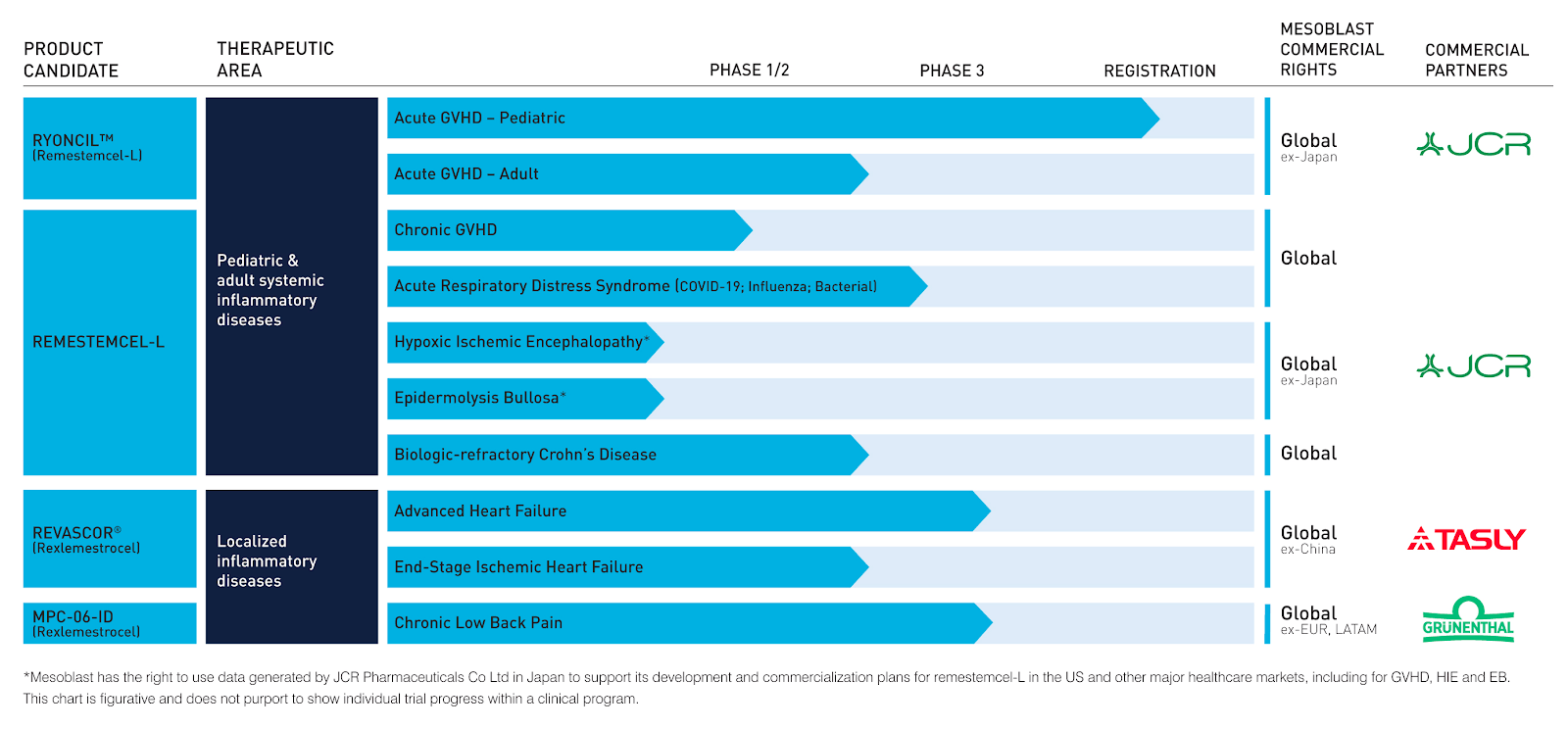
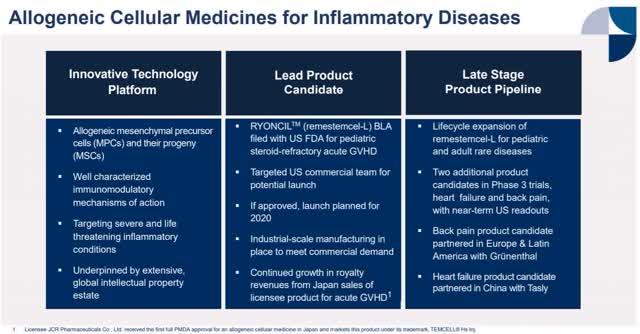
Ryoncil is currently under priority review by the us.

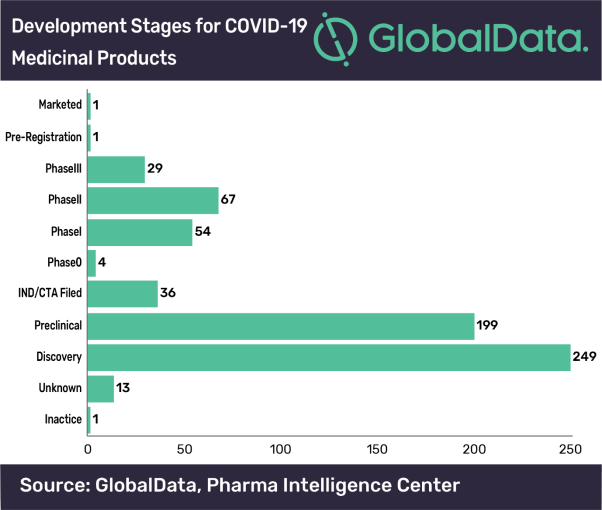
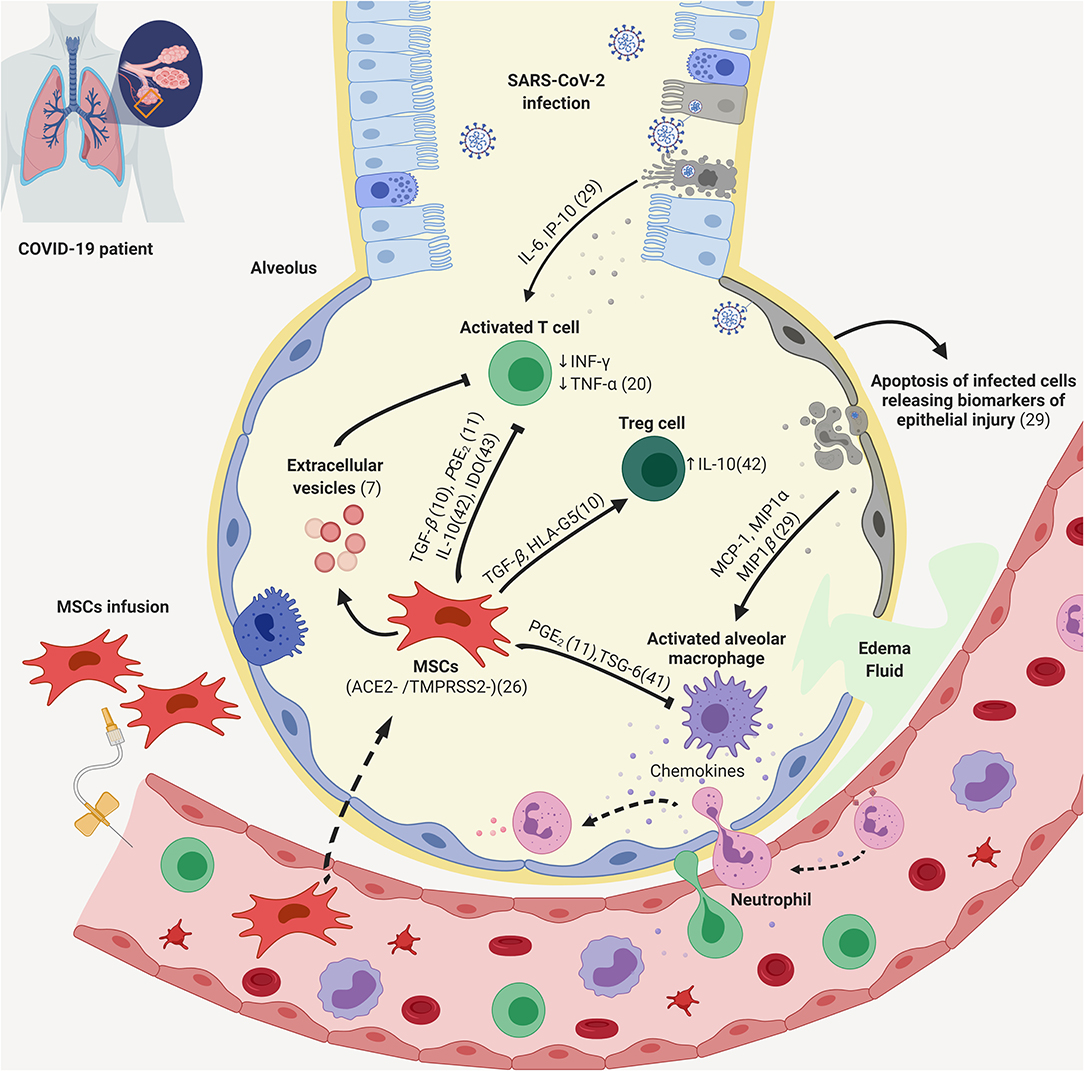
Mesoblast remestemcel l covid. Melbourne australia and new york based mesoblast limited announced that in a study of covid 19 patients with moderate to severe acute respiratory distress syndrome ards there was 83 survival with two intravenous infusions of the companys experimental allogeneic mesenchymal stem cell candidate ryoncil remestemcel l. 1 this is supported by recently published results from an investigator initiated clinical study conducted in. Mesoblasts allogeneic mesenchymal stem cell candidate remestemcel l showed exceptional promise when given to 12 ventilator dependent covid 19 patients with acute respiratory distress syndrome. Food and drug.
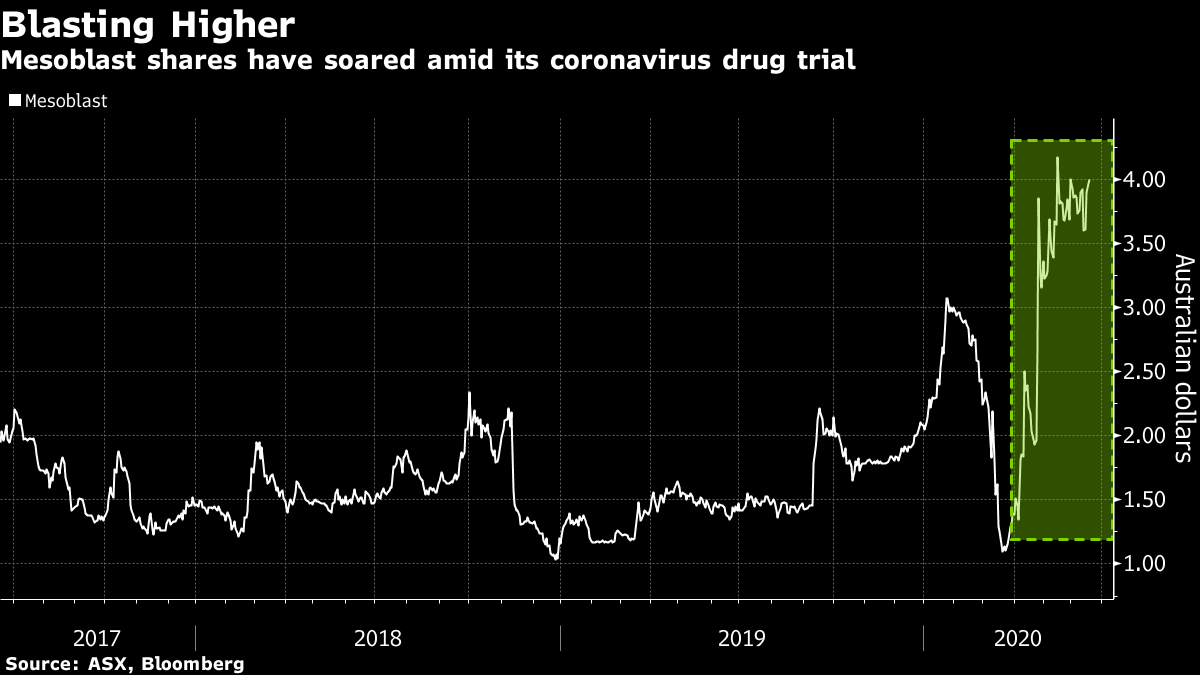
In april mesoblast received fda clearance for its ind application to treat patients with ards caused by covid 19 with intravenous infusions of remestemcel l nearly a month after disclosing march. Msb has received us food and drug administration investigational new drug ind clearance for its remestemcel l treatment to be used in covid 19 patients presenting with acute respiratory distress syndrome. Mesoblast chief medical officer fred grossman said there is a significant need to improve the dismal survival outcomes in covid 19 patients who progress to ards and require ventilators. Grossman notes the mortality rate in moderate to severe ards due to covid 19 can be as high as 80 per cent.
Remestemcel l has potential for use in the treatment of ards which is the principal cause of death in covid 19 infection. Remestemcel l has demonstrated safety efficacy and significant survival benefit in agvhd where inflammation is at the core similar to ards from covid 19 says mesoblast chief medical officer dr fred grossman. According to mesoblast chief medical officer dr fred grossman the fda ind approval now enables us based covid 19 patients with poor. Regenerative medicine company mesoblast asx.