Mesoblast Remestemcel L
According to mesoblast chief medical officer dr fred grossman the fda ind approval now enables us based covid 19 patients with poor.

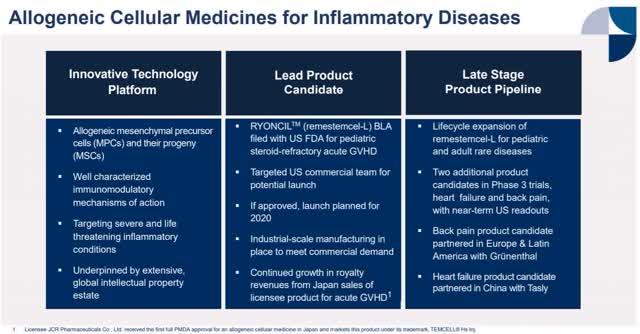
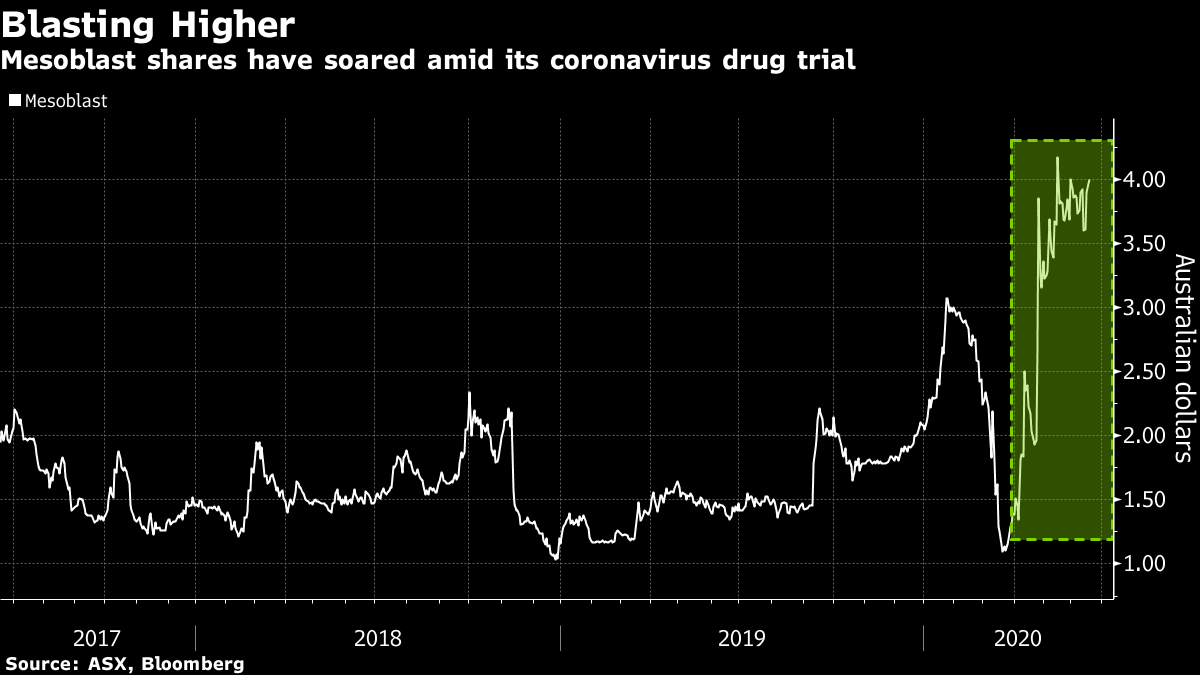
Mesoblast remestemcel l. Mesoblast is using its proprietary mesenchymal lineage cell technology platform to develop and commercialize innovative allogeneic cellular medicines to treat complex inflammatory diseases resistant to conventional standard of care. Mesoblast chief executive dr silviu itescu stated. Msb advanced remestemcel l drug could potentially boost the survival rate of critically ill covid 19 patients who have developed acute respiratory distress syndrome with initial results revealing an 83 survival rate for patients treated with the drug compared to 12. Remestemcel l is an intravenous adult human mesenchymal stem cell formulation prochymal being developed by mesoblast limited for the treatment of crohns remestemcel l mesoblast adisinsight either you have javascript disabled or your browser does not support javascript.
Msb has received us food and drug administration investigational new drug ind clearance for its remestemcel l treatment to be used in covid 19 patients presenting with acute respiratory distress syndrome. Mesoblast has filed a biologics license application to the united states food and drug administration fda to seek approval of its product candidate ryoncil remestemcel l for steroid. According to mesoblast 10 out of 12 covid 19 ventilator dependent patients with acute respiratory. Mesoblasts allogeneic mesenchymal stem cell candidate remestemcel l showed exceptional promise when given to 12 ventilator dependent covid 19 patients with acute respiratory distress syndrome.
Ryoncil remestemcel l is being developed for the treatment of acute graft versus host disease agvhd a potentially life threatening complication of an allogeneic bone marrow transplant bmt. Remestemcel l has two imminent major milestones the interim analysis in the ongoing phase 3 trial of remestemcel l in covid 19 patients with. Mesoblasts biologics license application bla for ryoncil for the treatment of children with steroid refractory agvhd has been. Remestemcel l is also being developed for other rare diseases.
Mesoblast announced data from a phase 23 trial evaluating remestemcel l an allogeneic mesenchymal stem cell product candidate in ventilator dependent covid 19 patients with moderate to severe.